Background: Gilteritinib is approved for patients (pts) with relapsed/refractory (R/R) FLT3-mutated acute myeloid leukemia (AML), based on findings from the phase 3 ADMIRAL trial (Perl AE, et al. N Engl J Med. 2019). A phase 3 trial, QuANTUM-R, demonstrated the benefit of quizartinib in pts with R/R AML with FLT3 internal tandem duplication (FLT3-ITD) mutations (Cortes JE, et al. Lancet Oncol. 2019). Although eligibility criteria across both studies were similar, QuANTUM-R was more stringent as to prior therapy intensity and remission duration, which potentially enriched for higher-risk pts. We sought to describe outcomes from ADMIRAL among pts who otherwise met eligibility for QuANTUM-R.
Methods: In this post-hoc analysis, a subset of pts from ADMIRAL were matched with R/R FLT3-ITD+ AML pts from QuANTUM-R on the basis of baseline characteristics and prior treatment criteria. Matched pts were either refractory to initial anthracycline-based chemotherapy or had relapsed ≤6 mos after achieving composite complete remission (CRc) with an anthracycline-based regimen.
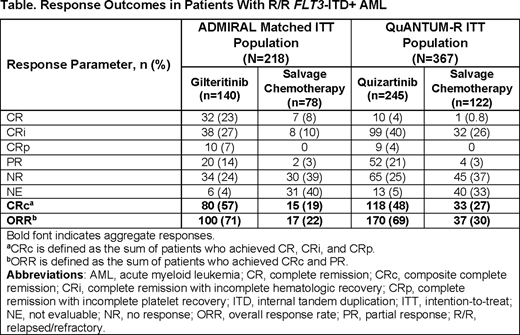
Results: Overall, 218 pts with R/R FLT3-ITD+ AML in the ADMIRAL trial (gilteritinib, n=140; salvage chemotherapy [SC], n=78) were matched with the QuANTUM-R intention-to treat (ITT) population (N=367; quizartinib, n=245; SC, n=122). Proportions of pts preselected for high-intensity SC were 66% (n=143/218) in the matched ADMIRAL ITT population and 77% (n=281/367) in the QuANTUM-R ITT populations. Demographic and baseline characteristics of the matched ADMIRAL ITT population and QuANTUM-R ITT population were similar. Median durations of exposure to gilteritinib and quizartinib were 3.8 mos and 3.2 mos, respectively, and median number of treatment cycles received were five and four, respectively. Rates of hematopoietic stem cell transplantation (HSCT) were similar in pts treated with gilteritinib (35%; n=49/140) or quizartinib (32%; n=78/245), as were the proportions of pts who resumed gilteritinib (23%; n=32/140) or quizartinib (20%; n=48/245) therapy post-HSCT.
Median overall survival (OS) in pts treated with gilteritinib or quizartinib was longer than that observed with SC. After a median follow-up of 17.4 mos, median OS was 10.2 mos with gilteritinib versus 5.6 mos with SC (hazard ratio [HR]=0.573 [95% CI: 0.403, 0.814]; one-sided nominal P=0.0008). After a median follow-up of 23.5 mos, median OS with quizartinib was 6.2 mos versus 4.7 mos with SC (HR=0.76 [95% CI: 0.58-0.98]; one-sided P=0.02). After censoring for HSCT, median OS was 9.3 mos with gilteritinib versus 5.5 mos with SC (HR=0.525 [95% CI: 0.356-0.775]; nominal one-sided P=0.0005), and 5.7 mos versus 4.6 mos with quizartinib versus SC, respectively (HR=0.79 [95% CI: 0.59, 1.05]; one-sided P=0.05). In both QuANTUM-R and matched ADMIRAL populations, the survival benefits of quizartinib and gilteritinib compared with SC were maintained across multiple subgroups, including high FLT3-ITD allelic ratio subsets.
Compared with SC, high CRc rates were observed in pts treated with either gilteritinib (57%; n=80/140) or quizartinib (48%; n=118/245). The complete remission (CR) rate with gilteritinib was 23% (n=32/140), whereas the CR rate with quizartinib was 4% (n=10/245) (Table). Median time to achieve CRc was 1.8 mos with gilteritinib and 1.1 mos with quizartinib, median duration of CRc was 5.5 mos with gilteritinib and 2.8 mos with quizartinib.
The safety profiles of gilteritinib and quizartinib were generally similar, though aspartate or alanine aminotransferase elevations (any grade) were more frequent with gilteritinib (41-44%) than quizartinib (≤13%), whereas neutropenia (14% vs 34%, respectively), fatigue (24% vs 39%, respectively), and prolonged QT intervals (9% vs 27%, respectively) were more frequent with quizartinib.
Conclusions: In pts with R/R FLT3-ITD+ AML and similar baseline characteristics, both gilteritinib and quizartinib were generally well tolerated and associated with improved survival and treatment response compared with SC. Responses to gilteritinib and quizartinib, as measured by CRc, were similar; blood count recovery varied between the two FLT3 inhibitors. Although cross-study comparisons have substantial limitations, the findings suggest that while remission is achieved faster with quizartinib, response may be more durable and survival potentially longer with gilteritinib.
Perl:Syndax: Consultancy, Honoraria; Leukemia & Lymphoma Society, Beat AML: Consultancy; Novartis: Honoraria, Other, Research Funding; Agios: Consultancy, Honoraria, Other; Jazz: Honoraria, Other; FORMA Therapeutics: Consultancy, Honoraria, Other; Daiichi Sankyo: Consultancy, Honoraria, Other: Writing/editorial support, travel costs for meetings, Research Funding; FUJIFILM Pharmaceuticals USA, Inc: Research Funding; New Link Genetics: Honoraria, Other; Arog Pharmaceuticals Inc: Other: uncompensated consulting, travel costs for meetings; Actinium Pharmaceuticals Inc: Consultancy, Honoraria, Research Funding; Biomed Valley Discoveries: Research Funding; Astellas: Consultancy, Honoraria, Other: writing/editorial support, travel costs for meeting presentations related to study, Research Funding; Bayer HealthCare Pharmaceuticals: Research Funding; AbbVie Inc: Consultancy, Honoraria, Other, Research Funding; Takeda: Honoraria, Other: Travel costs for meeting; Loxo Oncology Inc, a wholly owned subsidiary of Eli Lilly & Company: Consultancy, Honoraria, Other. Lu:Astellas: Current Employment. Fan:Astellas Pharma: Current Employment. Hasabou:Astellas Pharma: Current Employment. Berrak:Astellas: Current Employment. Tiu:Eli Lilly & Company: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Astellas Pharma Global Development: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal